Liezel M Gouws and Jan-Hendrik Groenewald
The safety of foods derived from genetically modified (GM) crops has been a subject of significant interest and discussion between technology developers, consumers and policy makers. “Are genetically modified organisms (GMOs) safe to eat?”, “Can they cause allergic reactions?”, and “How are the safety and quality of GM foods ensured?” are some of the questions that are often asked when GM foods are discussed. To answer these questions we should define exactly what GM crops are, how they differ from conventional crops and why they are treated / regulated differently to conventional food crops.
GM crops are those whose genetic make-up, i.e. their heritable traits, were modified in a specific and direct manner, using modern biotechnology techniques not dependent on traditional breeding techniques. This allows for the introduction of novel traits not previously associated with the particular crop. For example, GM crops currently cultivated have introduced insect resistance and / or herbicide tolerance traits while new GM crops currently under development are more drought tolerant and have improved nutritional profiles. This novelty aspect associated with GM crops is the reason why they, and the foods derived from them, are specifically regulated and extensively evaluated before commercial production is allowed. Conventional foods are considered safe, based purely on their history of safe use. In lieu of such historic information GM crops undergo formal, rigorous environmental and food safety assessments to ensure their safety. This article aims to shed more light on the regulatory and technical frameworks used in South Africa to ensure the development of safe and sustainable GM products.
Regulatory Framework
A number of national and international regulations govern the use of GMOs. The primary aim of these regulations is to ensure that any activities with GMOs are assessed with regard to their potential risks to human health and the environment. Organisations such as the Organisation for Economic Cooperation and Development (OECD), the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO) and the International Life Science Institute (ILSI) have developed broad consensus documents for the safety assessment of GM foods. The Codex Alimentarius Commission, a joint FAO / WHO body, is responsible for compiling the standards, codes of practice, guidelines and recommendations that represent the Codex Alimentarius, also known as the international food code. South Africa is a member of the Codex Alimentarius Commission and accordingly follows the Codex principles and guidelines for the evaluation of the safety of food and feed derived from GMOs.
Nationally, GMOs are primarily governed by the GMO Act (Act 15 of 1997), which provides measures to promote the responsible development and use of GMOs in South Africa. The National Department of Health is a member of the Executive Council of the GMO Act, the ultimate decision making body on GMOs in South Africa and has, in this capacity, developed a national guideline on the Food Safety and Risk Assessment of Genetically Modified Organisms and Products Thereo” in line with Codex Alimentarius requirements. Applicants must submit all the relevant data required for the food safety assessment (discussed later) including full descriptions of the test procedures, methods, results and conclusions. In general, the assessment of the safety of GMOs intended for food or feed is conducted on a case-by-case basis. Different GM organisms have different genes inserted in different ways, making it impossible to make general statements or conclusions regarding all GM crops.
Risk Assessment
Risk assessment is a formalised process whereby the possible risks associated with a particular action or product are objectively evaluated in a manner which clearly considers assumptions and uncertainties. Before GM foods emerged, food safety assessments tended to focus on single, potentially hazardous chemical entities associated with foods. GM foods, however, are assessed as a whole and represent complex, variable mixtures of components, which meant that a new risk assessment approach had to be developed for them. Consequently, international consensus was reached on using a comparative approach, i.e. comparing the novel food to its conventional counterpart, and the concept of substantial equivalence. This approach allows risk assessors to include historic information in their consideration, related to foods which have long been safely consumed by humans, to establish whether the products are equivalent, i.e. have similar risk profiles. It is important to realise that the goal of this approach is not to try and establish “absolute safety” but rather to consider whether the GM food is at least as safe as its conventional counterpart ̶ possible significant differences are therefore an important focus of any risk assessment. This structured, stepwise process can be formalised as a consensus, food safety risk assessment framework as proposed by Codex and employed by the Department of Health.
Framework for the Safety Assessment of Foods Derived from GM Crops
The four facets of a food safety assessment focus respectively on the host plant (the conventional counterpart), the organism which donated the novel trait, the novel genetic material or genetic intervention itself and, finally, the resulting GM plant.
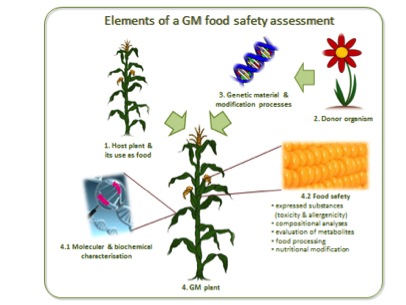
Generally speaking, GM foods are assessed on a case-by-case basis and no distinction is made between food crops and crops intended only for animal feed, i.e. all are considered part of the human food chain. Ultimately, food safety assessments are conducted to provide science-based evidence that foods derived from GM crops are safe for consumption. Similarly, the environmental safety and socio-economic sustainability of a GM crop have to be assessed and approved before it can be released for commercial production.
Labelling of GM Foods in South Africa
The Department of Health has had GM food labelling regulations in place since 2004. However, none of the GM products currently available in South Africa have triggered these regulations, as they are aimed at informing consumers on safety-related issues, such as the introduction of new allergens and changes in nutritional value and / or preparation techniques. In other words, based on the outcomes of the risk assessment process as described above, all GM foods currently on the South African market are considered equivalent to their conventional counterparts and are therefore not required to be labelled under these regulations. More recently though (October 2011), regulations under the Consumer Protection Act (Act 68 of 2008) have enforced the mandatory labelling of all foods containing GM ingredients or which have been produced using GMOs, irrespective of the outcome of the risk assessment. Accordingly, these regulations only address vaguely defined consumer preference issues and do not relate to any food (or environmental) safety aspects. In addition, many questions regarding the implementation of these regulations remain at this early stage, as they contain several ambiguities and technical inaccuracies, which will probably only be resolved through case law or subsequent amendments.
Conclusion
Generally, the safety of GMOs has been assessed since 1982 and a huge body of peer-reviewed, scientific literature attests to the safety of the commercially available GM crops, which have been subjected to a thorough risk assessment process before release was approved. To date, after 17 years of large scale commercial production, no adverse effects on human health due to the consumption of GM foods have been documented. In addition, South Africa has a rigorous regulatory framework, which has been fully functional for more than 14 years and which will only approve a GM crop for commercial release if all the stringent safety standards, including post-release risk management requirements, are met. To conclude, foods derived from approved GM crops are as safe as their conventional counterparts. Consumers can rest assured that GM crops approved for commercial release in South Africa have undergone extensive safety assessments and that they have met all the stringent requirements set by the national regulatory framework.
Relevant Links
Department of Agriculture, Forestry and Fisheries www.daff.gov.za
Department of Health www.doh.gov.za
Codex Alimentarius www.codexalimentarius.net
European Food Safety Authority www.efsa.europa.eu
Biosafety South Africa www.biosafety.org.za
Biosafety South Africa is a national biosafety service platform that promotes the biosafety and sustainability of biotech products through the delivery of value adding services and investment in strategic biosafety research. It operates under the auspices of the Technology Innovation Agency and the Department of Science and Technology to support innovation in biotechnology.
Dr Liezel Gouws is a Project Manager at Biosafety South Africa and is responsible for the coordination, management and sourcing of biosafety projects, regulatory dossiers and resources. She has a PhD in plant biotechnology and, prior to joining Biosafety South Africa, she completed postdoctoral fellowships at the Institutes for Plant Biotechnology and Wine Biotechnology at Stellenbosch University. Her research career focused mainly on plant growth analysis, specifically plant gene expression, proteomics and metabolomics.
Dr Hennie Groenewald is the Executive Manager of Biosafety South Africa and is responsible for the strategic management, business development and service delivery of the national service platform. He has more than 20 years’ experience in research and development, teaching, project management and business development. He is currently also a member of the National Biotechnology Advisory Committee (NBAC), the Medicines Control Council (MCC) and the International Society for Biosafety Research (ISBR).
*This article first appeared in FST (South African Food Science and Technology) magazine and is reprinted by kind permission.
IUFoST Scientific Information Bulletin (SIB)
FOOD FRAUD PREVENTION